CELLS
Wet Cells:
|
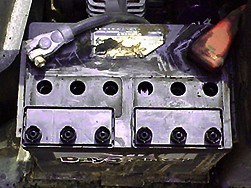
This battery is from a very large mower. A wet cell like this is
found on most cars. |
What is a wet cell: |
How a wet cell works:
In a wet cell, the zinc electrodes and the sulfuric acid react together. The
reaction causes electrons to collect on the zinc electrode. The zinc electrode
gets a negative charge, while the copper electrode receives a positive charge.
When the zinc and copper and connected by a wire, electrons move through the
wire. Electrons from the negative zinc electrode push each other through the
wire to the positively charged copper electrode. The movement
from the electrons produce an electric current.
Dry Cells
|
|
|
What is a dry cell:
If you tipped over a wet cell, the electrolyte would spill out. Wet cells
cannot be used in flashlights or in other things that are carried around. For
these objects, dry cells are used. Dry cells are another kind of electric cell.
Dry cells can be made very small, and are carried around easily. Flashlights,
transistor radios, and many toys use dry cells.
How a dry cell works:
A dry cell is not completely dry. It has moist electrolyte inside it. The
entire outside case of a dry cell is the negatively charged electrode, which is
composed of zinc. Part of the negative electrode sticks up at the top of the
cell, near the edge. There is a place to connect a wire to the zinc, which is
called the negative terminal. The positive electrode in a dry cell is a carbon
rod. It goes down the center of the dry cell. The positive terminal is attached
to the top of the carbon rod.
A chemical reaction between the electrolyte and the zinc electrode
makes electrons collect on the zinc strip. When the positive and negative
terminals of the dry cell are connected by means of the wire, electrons move
through the wire from the negative terminal to the positive terminal. Electric
current always moves from a negative terminal to a positive terminal.
What happens to the